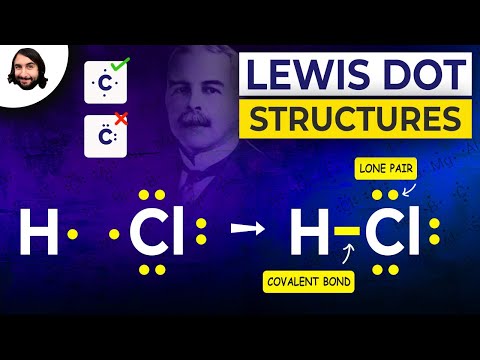
What does a dot in a dot diagram stand for?
Home › Articles, FAQ › What does a dot in a dot diagram stand for?Explanation: A single dot represents the one electron. A dash typically represents 2 electrons, and typically represents a covalent bond, i.e. shared electron density between two positively charged atomic nuclei.
Q. What is an atom kid definition?
The atom is the basic building block for all matter in the universe. Atoms are extremely small and are made up of a few even smaller particles. The basic particles that make up an atom are electrons, protons, and neutrons. They can change and undergo chemical reactions, sharing electrons with other atoms.
Table of Contents
Q. Are atoms energy?
Each atom has a set of energy levels associated with it. All of the atoms of a particular element have the same set of energy levels, but every element has a unique set of energy levels associated with its atoms. Knowing the energy levels identifies the element.
Q. How do atoms become stable?
Some atoms become more stable by gaining or losing an entire electron (or several electrons). When they do so, atoms form ions, or charged particles. Electron gain or loss can give an atom a filled outermost electron shell and make it energetically more stable.
Q. How is the structure of an atom linked to its position in the periodic table?
The electronic configuration of an element is related to its position on the periodic table. the number of electrons in all shells of an element is represented in the periodic table as the element’s atomic number.
Q. What is the relationship between the periodic table and atoms?
The arrangement of the periodic table leads us to visualize certain trends among the atoms. The vertical columns (groups) of the periodic table are arranged such that all its elements have the same number of valence electrons. All elements within a certain group thus share similar properties.
Q. How is an atom structure?
Atoms consist of three basic particles: protons, electrons, and neutrons. The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and contain the electrons (negatively charged).
Want to go more in-depth? Ask a question to learn more about the event.